
The Sridharan Lab is currently interviewing for postdoctoral trainees and research interns.
Please direct inquiries to Dr. Sridharan at rsridharan2@wisc.edu

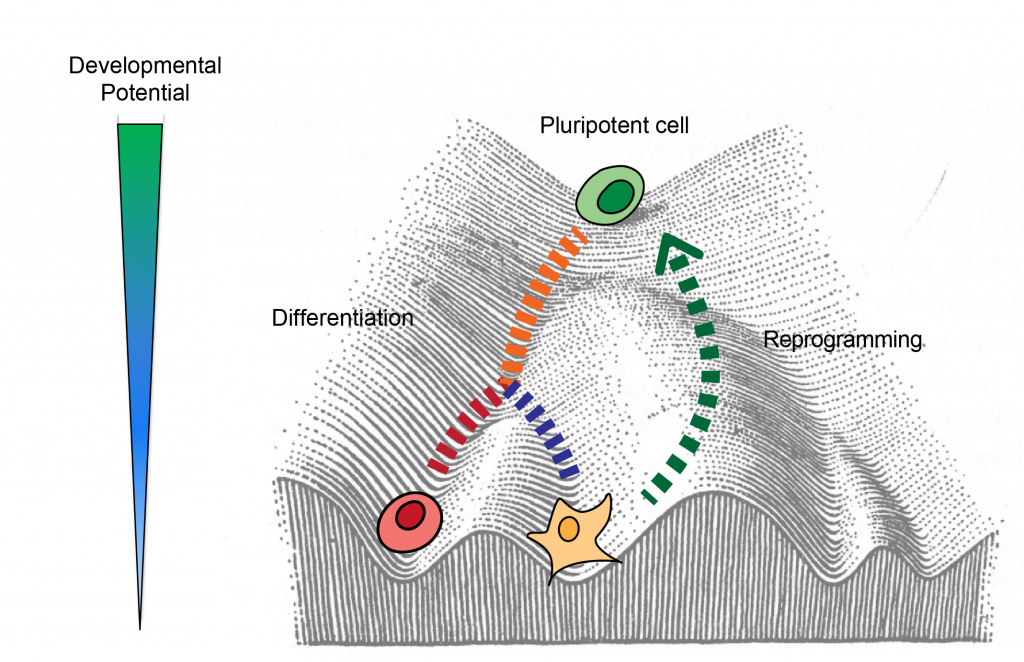
Our lab is focused on investigating how chromatin modifications that control gene expression and can be transmitted across cell divisions control the establishment , maintainence and disruption of cell identity in development and disease. For this purpose we use genomic and gene editing techniques wide techniques to query the epigenome and transcriptome at the population and single cell level. Ultimately we are interested in gaining fundamental insights into cell fate and applying the information to derive cells with desired properties for regenerative therapy.
 Feedback, questions or accessibility issues:
Feedback, questions or accessibility issues: